Introduction
The prognostic value of secondary ontogeny in newly diagnosed acute myeloid leukemia (AML) is based on data from patients (pts) treated with intensive chemotherapy. Secondary ontogeny-defining mutations have been integrated into recently revised diagnostic and prognostic criteria, irrespective of initial therapy. We conducted a retrospective cohort study of pts treated with hypomethylating agents (HMA) +/- venetoclax (VEN) in order to determine the prognostic value of molecular ontogeny in AML treated with low-intensity regimens and evaluate whether the observed benefit of VEN is distinct for each ontogeny.
Methods
We included all pts with newly diagnosed AML who were treated with either HMA (HMA monotherapy or HMA+non-VEN drug) or HMA+VEN at Dana Farber Cancer Institute between 2014 and 2022. We defined three ontogeny groups in hierarchical order (Lindsley, Blood 2015) based on the presence of a TP53 mutation (“ TP53 group”), the presence of ≥1 secondary ontogeny mutation without concurrent TP53 mutation (“secondary group”), and all others (“de novo” group). Overall 314 pts were included: 111 (35%) in the TP53 group (60 with HMA+VEN, 51 with HMA); 115 (37%) in the secondary group (68 with HMA+VEN, 47 with HMA); 88 (28%) in the de novo group (38 with HMA+VEN, 50 with HMA). No significant differences in baseline characteristics were observed between pts treated with HMA+VEN vs HMA. Composite complete response (cCR) was defined as Complete response (CR) plus CR with incomplete recovery (CRi) and was evaluable in 252/314 (80%). Overall survival (OS) was compared by a log-rank test. Univariate and multivariable Cox regression models were fitted to assess the effect of covariates on OS with allogeneic hematopoietic cell transplant (HCT) as a time-varying covariate.
Results
Overall, the cCR rate (49% vs 28%, p=0.001), HCT rate (17% vs 8%, p=0.018), and OS (median OS 9.9 [95% confidence interval (CI) 8-13] vs 7.4 [95% CI 6-10], p=0.018) were significantly better in pts treated with HMA+VEN compared with those treated with HMA.
To determine whether the benefit of VEN was different based on molecular ontogeny, we assessed outcomes in each group separately. In patients with TP53 mutations, there was no difference in cCR rate between pts treated with HMA+VEN (30%) vs HMA (25%), p=0.82. Conversely, the cCR rates were significantly higher with HMA+VEN vs HMA in those in the secondary (50% vs13%, p<0.001) and de novo groups (50% vs 22%, p=0.034). The HCT rates between HMA+VEN vs HMA were higher in the secondary group (24% vs. 6%, p=0.02) and were similar in the TP53 and de novo groups (15% vs 8%, p=0.38 and 11% vs 10%, p> 0.99, respectively).
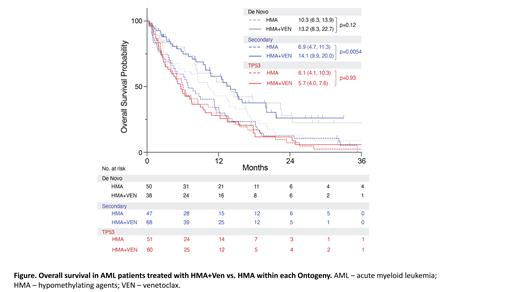
In the TP53 group, OS was similar in those treated with HMA+VEN vs HMA (median OS 5.7 months [95% CI 4-8] vs 6.1 [95% CI 4-10], respectively, p=0.93). In contrast, pts in the secondary group treated with HMA+VEN vs HMA had significantly better OS (median OS 14.1 months [95% CI 10- 20] vs 6.9 [95% CI 5-11], p=0.0054, Figure). In the de novo group, OS was not significantly different between HMA+VEN ( median OS 13.2 months [95% CI 8-23]) vs HMA (median OS 10.3 [95% CI 6-14]), p=0.12.
To verify the prognostic significance of VEN in each ontogeny and adjust for potential confounders, we performed regression models for each ontogeny separately. HMA+VEN vs HMA was associated with significantly improved survival only in the secondary group (secondary: hazard ratio [HR] 0.59, 95% CI 0.38-0.94, p=0.025; TP53: HR 1.19, 95% CI 0.78-1.81, p=0.42; de novo: HR 0.67, 95% CI 0.40-1.12, p=0.13). HCT was associated with improved OS across all groups ( TP53: HR 0.17, 95% CI 0.07-0.42, p<0.001; secondary: HR 0.40, 95% CI 0.18-0.89, p=0.026; de novo: HR 0.14, 95% CI 0.03-0.58, p=0.0067).
Among all pts treated with HMA+VEN (n=166), the OS was similar between de novo and secondary groups (p=0.92); and both groups had better OS compared to the TP53 group (comparison vs de novo p=0.007; comparison vs secondary p<0.001). These results were verified in a regression analysis, adjusting for age, HCT, and disease characteristics.
Conclusion
In newly diagnosed AML patients, treatment with HMA+VEN had a particular benefit in those with secondary mutations, with better response and HCT rates, and prolonged OS compared with HMA. Consequently, outcomes were similar in pts with genetically defined secondary and de novo disease. In pts with TP53 mutations, treatment with HMA+VEN did not improve any clinical outcomes compared with HMA.
Disclosures
Garcia:Gilead: Consultancy; Bristol Myers Squibb: Consultancy; AbbVie: Consultancy, Research Funding; New Wave: Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy; Pfizer: Research Funding; AstraZeneca: Research Funding; Prelude: Research Funding; Servier: Consultancy. Chen:Rigel Pharmaceuticals: Consultancy, Honoraria; Abbvie Pharmaceuticals: Research Funding. Luskin:Novartis: Research Funding; Novartis: Honoraria; Pfizer: Honoraria; Jazz: Honoraria; AbbVie: Research Funding. Stahl:Kymera: Membership on an entity's Board of Directors or advisory committees; Boston Consulting: Consultancy; GSK: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees; Clinical care options: Other: GME activity ; Rigel: Membership on an entity's Board of Directors or advisory committees; Dedham group: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: GME activity ; Haymarket Media: Other: GME activity ; Curis Oncology: Other: GME activity . Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. DeAngelo:Kite: Honoraria; Novartis: Research Funding; AbbVie: Research Funding; Blueprint: Honoraria; Blueprint: Research Funding; Incyte: Honoraria; Servier: Honoraria; Amgen: Honoraria; Autolus: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Jazz: Honoraria; GlycoMimetics: Research Funding; Gilead: Honoraria; Takeda: Honoraria. Stone:Jazz: Consultancy; Lava Therapeutics: Consultancy; BerGenBio: Consultancy; GSK: Consultancy; Rigel: Consultancy; Hermavant: Consultancy; Aptevo: Other: DSMB; Kura One: Consultancy; Cellularity: Consultancy; CTI Biopharma: Consultancy; Takeda: Other: DSMB; Abbvie: Consultancy; Syntrix: Other: DSMB; AvenCell: Consultancy; Epizyme: Other: DSMB; Ligand Pharma: Consultancy; Amgen: Consultancy. Lindsley:Bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Qiagen: Consultancy; Sarepta Therapuetics: Consultancy; Verve Therapuetics: Consultancy; Jazz Pharmaceuticals: Consultancy; Vertex Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal